WATCHMAN FLX™ Pro Left Atrial Appendage Closure Device
Built on the proven safety profile of the WATCHMAN FLX Device1, the WATCHMAN FLX Pro Device is designed to enhance the healing process and optimize the therapy for more patients.2
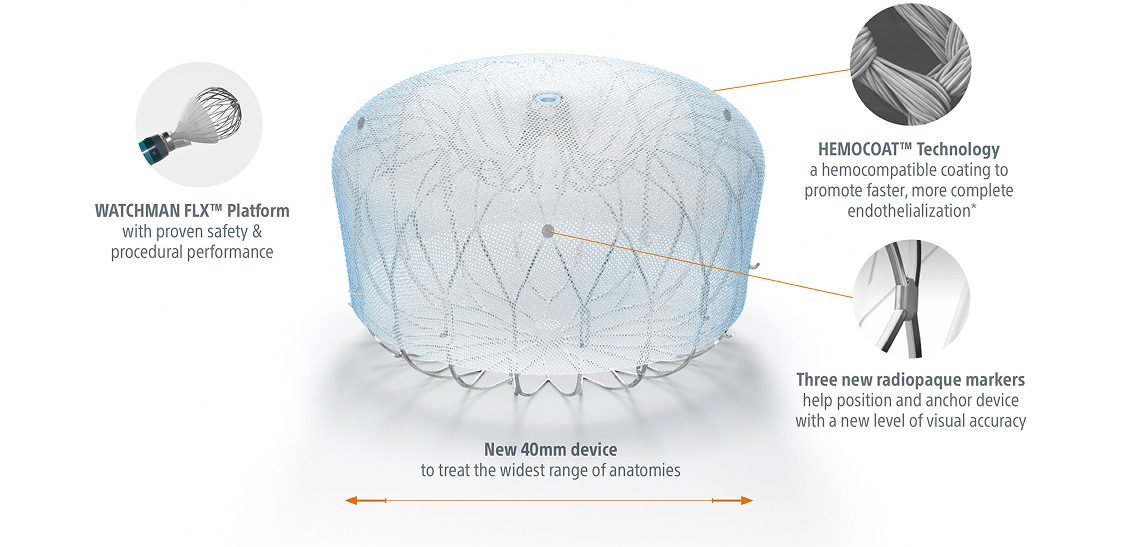
*Enhanced healing demonstrated in pre-clinical model with no anticoagulation
How we can help you and your program

HAWKEYE
HAWKEYE is an online solution to help you facilitate LAAC patient workflow. HAWKEYE helps you understand program performance and patient outcomes, making it easier to optimize operational efficiencies and streamline patient coordination.

Healthcare Solutions Team
Healthcare Solutions is a team of former hospital administrators who help LAAC programs identify opportunities to accelerate program success and development. The Calibration Program identifies solutions to optimize LAAC programs.
Learn more about WATCHMAN FLX Pro
Access resources
Access WATCHMAN FLX Pro resources by visiting our WATCHMAN Download Center
Rep visit
Connect with a local representative to learn more about the WATCHMAN FLX Pro
References
1. Kar, S., et al, Circulation, 2021
2. Saliba et al. Enhanced Thromboresistance and Endothelialization of a Novel Fluoropolymer-Coated Left Atrial Appendage Closure Device, JACC: Clinical Electrophysiology, 2023.

