
Over 600.000 AFib patients globally have left blood thinners behind, replacing OACs with the WATCHMANᵀᴹ Implant.¹
Proven1
600.000+
patients treated
20+
years clinical experience
Safe2
99%
implant success rate*
0.5%
major adverse event rate†

Effective2
96.2%
of patients discontinued OACs at 45 days
Protect your patients: stroke risk reduction without the risks of OACs
Learn more about the WATCHMANᵀᴹ Implant and hear from cardiologists on this safe, OAC alternative for stroke risk reduction.
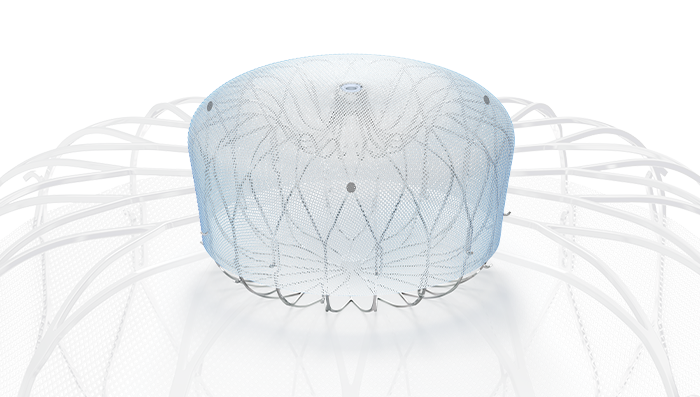
Atrial fibrilation and stroke prevention
Learn about the difference of the WATCHMANᵀᴹ implant
Access resources
Access WATCHMANᵀᴹ resources in the referring HCP download centre
Talk to someone
Connect with a local representative to learn about the WATCHMANᵀᴹ Implant
*Procedure success defined as successful delivery and release of a WATCHMAN FLX Device into the LAA.
†Occurrence of one of the following events between the time of implant and within 7 days following the procedure or by hospital discharge, whichever is later: all-cause death, ischemic stroke, systemic embolism, or device or procedure related events requiring open cardiac surgery or major endovascular intervention.
References:
1. Represents all WATCHMAN models.
2. Kar, S., et al, Primary Outcome Evaluation of the Next Generation LAAC Device: Results from the PINNACLE FLX Trial, Circulation, 2021.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.

