References
1. Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010.
2. World Stroke Organization & World Heart Federation. Understanding atrial fibrillation and stroke. https://www.world-stroke.org/assets/downloads/STROKE_RISK_AND_PREVENTION_LEAFLET_-_ATRIAL_FIBRILLATION-EN.pdf
3. Dudziñska-Szczerba K, et al. Association Between Left Atrial Appendage Morphology and Function and the Risk of Ischaemic Stroke in Patients with Atrial Fibrillation. Radcliffe Cardiology, 2022. https://pubmed.ncbi.nlm.nih.gov/35846423/
4. Data on file with Boston Scientific. Atrial Fibrillation Patients survey. The Harris Poll on behalf of Boston Scientific and StopAFib.org; 2018:1-64.
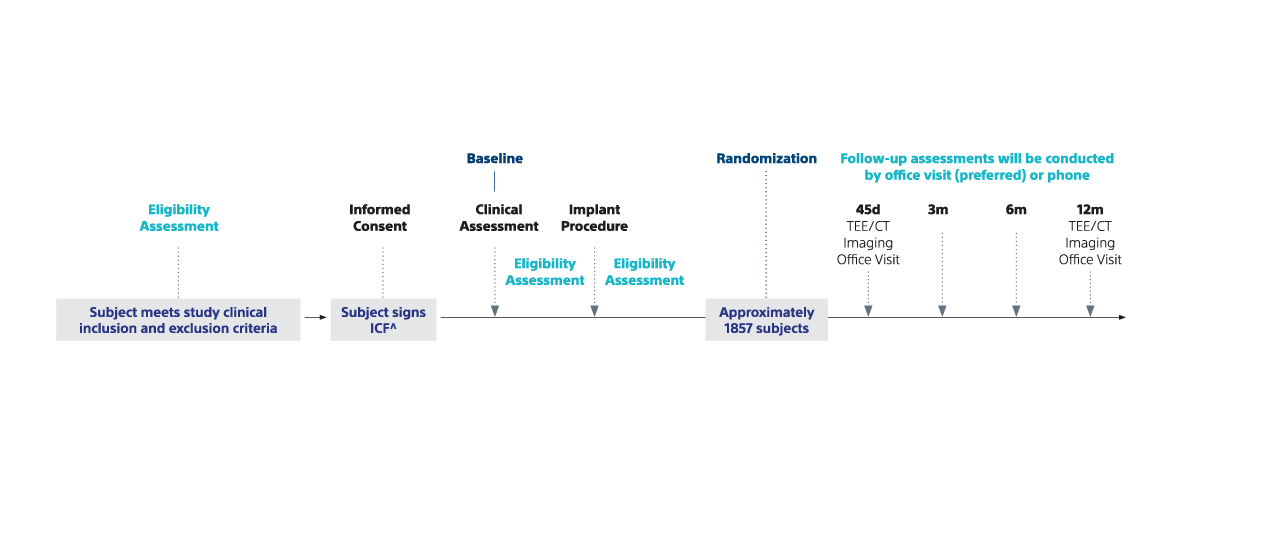
5. BSC data on file. 2025
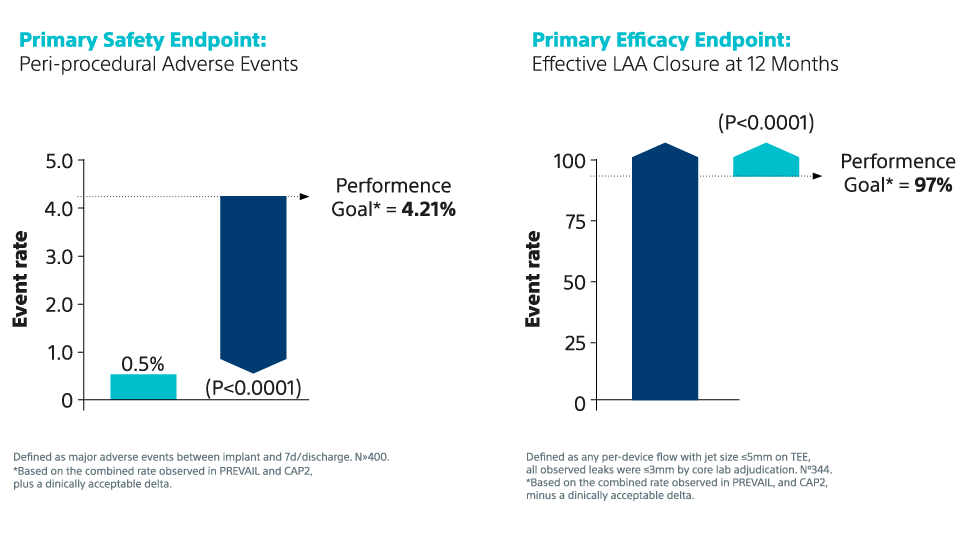
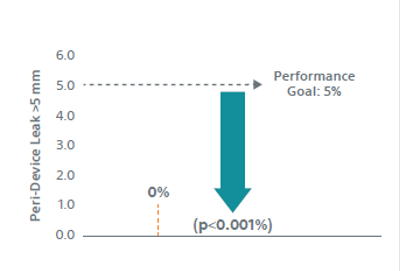
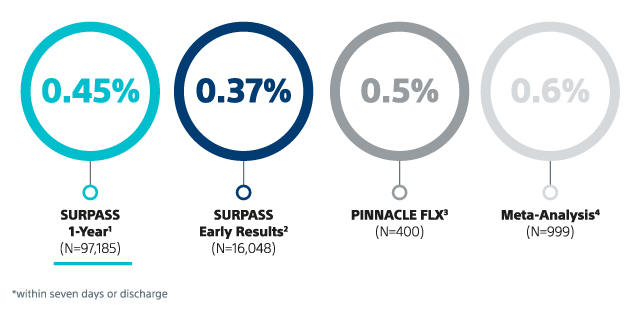
6. Kar S, et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation, 2021; 143(18): 1754-62. DOI: 10.1161/CIRCULATIONAHA.120.050117
7. Wazni OM, et al. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation. N Eng J Med 2025; 392(13): 1277-87. DOI: 10.1056/NEJMoa2408308
8. Kanj M, et al. Safety and Performance of a Novel Access System for LAAO: A Sub-analysis of the HEAL-LAA Study. Moderated Abstract, TCT 2024.
9. Doshi SK, et al. Two‐Year Outcomes With a Next‐Generation Left Atrial Appendage Device: Final Results of the PINNACLE FLX Trial. JAHA 2023; 12(4). Epub: doi: 10.1161/JAHA.122.026295.
10. Alli O, et al. Primary Safety and Efficacy Endpoints of the HEAL-LAA Post-Approval Clinical Study. LAAO Innovations Session, TCT 2024.
11. Kapadia S.R., et al. Outcomes With the WATCHMAN FLX in Everyday Clinical Practice From the NCDR Left Atrial Appendage Occlusion Registry. SURPASS RWD. Circulation: Cardiovasc. Interv. 2024; 17(9): e013750. DOI: https://doi.org/10.1161/CIRCINTERVENTIONS.123.013750
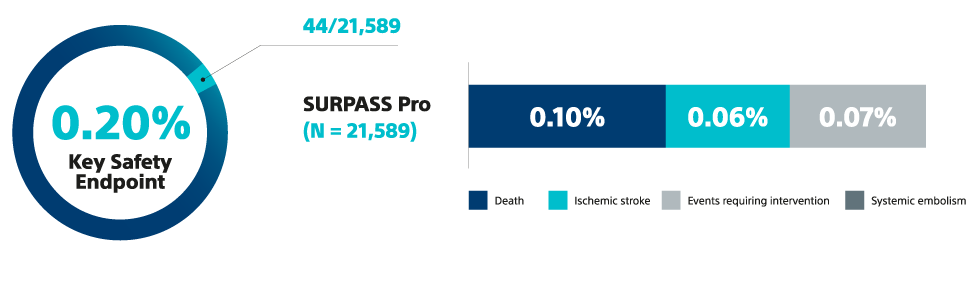
12. Piccini J et al. Outcomes with WATCHMAN FLX Pro in Everyday Clinical Practice: Early Results from SURPASS Pro, HRS 2025.
13. Kar S., et al. Rationale and Design of a Randomized Study Comparing the Watchman FLX Device to DOACs in Patients with Atrial Fibrillation, American Heart Journal (2023), doi: https://doi.org/10.1016/j.ahj.2023.05.022
14. National Library of Medicine. SIMPLAAFY Clinical Trial (SIMPLAAFY). 2025-10-15. https://www.clinicaltrials.gov/study/NCT06521463?tab=history&a=17#version-content-panel