The SURPASS early results analysis
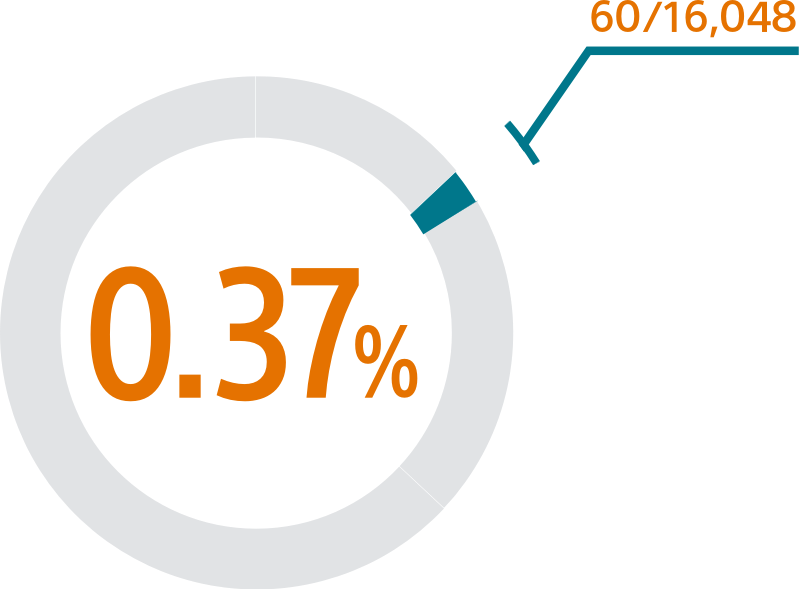
The SURPASS early results analysis of the NCDR-LAAO Registry™ reinforces the outstanding safety of WATCHMAN FLX with a 0.37% major procedural adverse event rate within 7 days or hospital discharge and 98% procedural success rate in > 16,000 real-world NVAF patients.1
Study design:
- SURPASS assesses the safety and efficacy outcomes in patients in the NCDR-LAAO Registry that received a commercial WATCHMAN FLX device
- This SURPASS analysis includes 16,048 patients receiving a WATCHMAN FLX device between August 5, 2020 and March 31, 2021
- SURPASS will continue to assess WATCHMAN FLX patients included in the NCDR-LAAO Registry from August 2020 through August 2022 and will follow these patients through 2 years post-implant
Mean baseline patient characteristics (N=16,048)
- Average age/years: 76.1 ± 7.9
- CHA2DS2-VASc: 4.8 ± 1.5
- HAS-BLED: 2.4 ± 1.0
- 40% women
- 62% with prior clinically relevant bleeding event
Safety endpoint
Composite of all-cause death, ischemic stroke, systemic embolism, or device/procedure-related events requiring open cardiac surgery or major endovascular intervention between device implantation and seven days or hospital discharge, (whichever is later).
SURPASS demonstrated 0.37% major procedural adverse event rate within 7 days or hospital discharge in 16,048 commercial patients and confirmed the trusted safety profile of WATCHMAN FLX in real-world clinical practice setting.
SURPASS data reinforces WATCHMAN FLX procedural success with 98% of patients implanted (N=16,048/16,446)1 across nearly all anatomies in a real-world setting, confirming that WATCHMAN FLX real world experience replicates clinical trial outcomes.
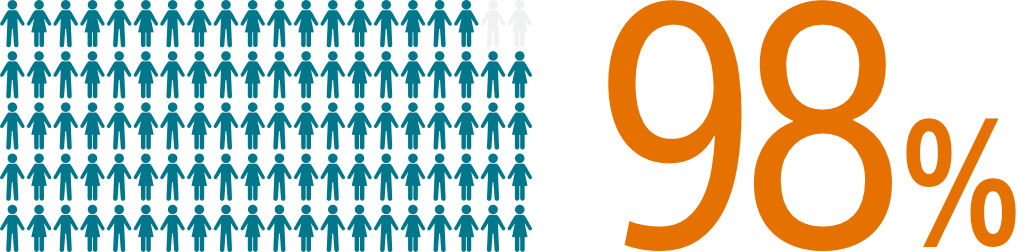
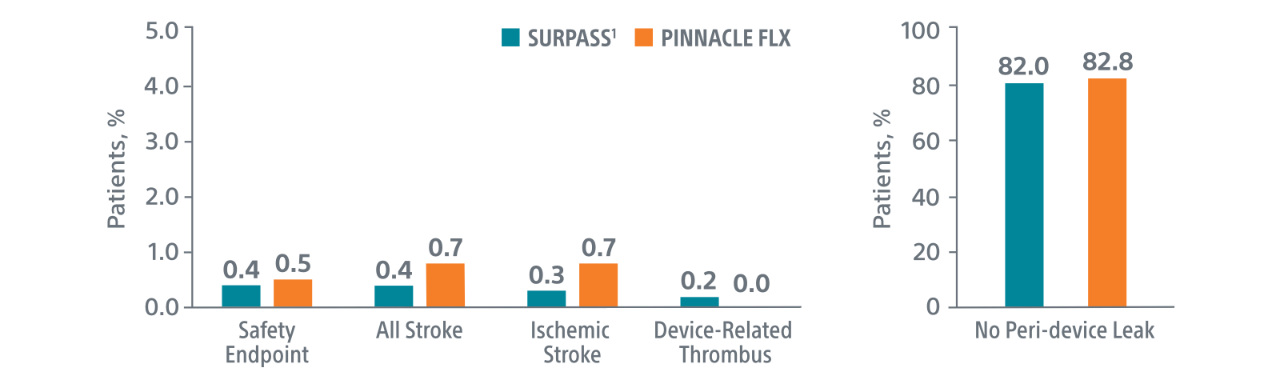
The early results SURPASS Data reinforces the excellent safety profile WATCHMAN FLX demonstrated in the PINNACLE FLX trial, with over 16,000 real-world WATCHMAN FLX patients analyzed.
Comparison with PINNACLE FLX2
45-day outcomes
The SURPASS 1 Year Outcomes analysis of the NCDR-LAAO Registry™ now includes the largest number of commercial WATCHMAN FLX™ Implant patients to date.
References
1. Late Breaking Clinical Trial at CRT 2022, Presented by Dr. Samir Kapadia.
2. Kar, Circulation 2021.
