How WATCHMAN works
In non-valvular atrial fibrillation (AFib), >90% of stroke-causing clots that come from the left atrium are formed in the left atrial appendage (LAA).1
The WATCHMAN Implant is a minimally invasive, one-time procedure designed to reduce the risk of strokes that originate in the LAA.
Permanent implant
 Minimally invasive
Minimally invasive
Minimally invasive
1 day or less average hospital stay
A one-time procedure that delivers a lifetime of stroke risk reduction, without oral anticoagulant (OAC) bleed risk.
Learn how the WATCHMAN FLX Pro Implant works.
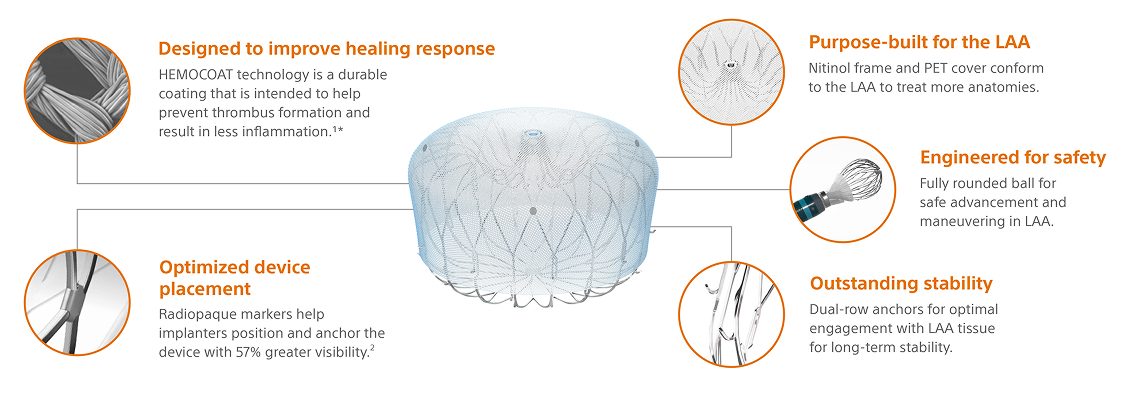
WATCHMAN FLX Pro Implant design
Built on the proven performance of WATCHMAN FLX, WATCHMAN FLX Pro is the latest WATCHMAN device with features designed to promote faster healing.2
WATCHMAN FLX Pro is available in six sizes to treat a variety of anatomies
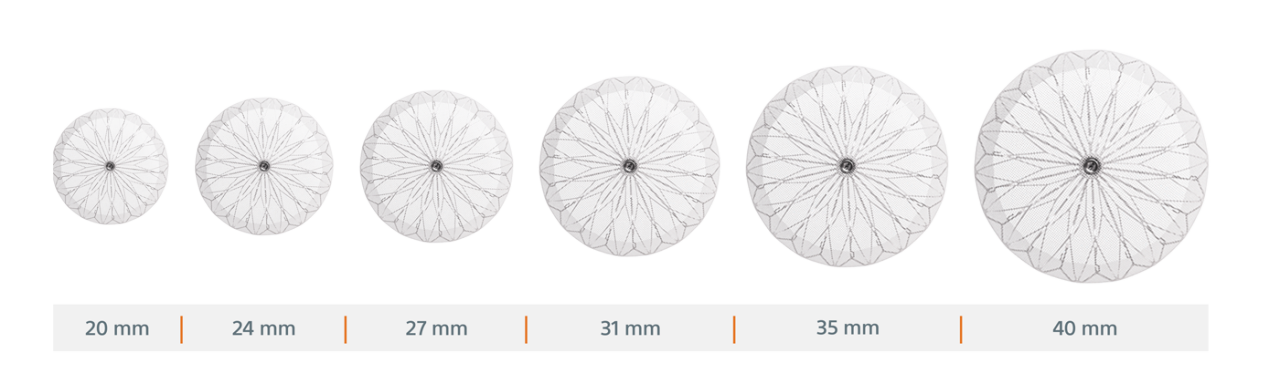
Note: Devices not shown to scale.
WATCHMAN LAAC Implant procedure overview

Step 1: Access
Using a standard percutaneous technique, a guidewire and vessel dilator are inserted into the femoral vein. The implant procedure is performed with fluoroscopy and transesophageal echocardiography (TEE).

Step 2: Cross
The interatrial septum is crossed using a standard transseptal access system. The access sheath is advanced over the guidewire into the left atrium and then navigated into the distal portion of the LAA over a pigtail catheter.

Step 3: Deploy
The WATCHMAN Implant is deployed and released in the LAA.

Step 4: Heal
Heart tissue grows over the implant and the LAA is permanently sealed. Patients will then follow the post-implant drug regimen as prescribed by their physician.

Step 5: Protect
The implant is fully endothelialized.
Flexibility to choose the appropriate post-implant drug regimen
The WATCHMAN Implant provides the flexibility to select the ideal drug regimen that is best for your patient with clinical outcomes that support safety and efficacy in preventing thrombosis and consequent stroke.
As always, you should exercise clinical judgment based on individual patient characteristics in determining the most appropriate use of anti-thrombotic drugs for the post-implant medication regimen.

Post-implant drug regimen options
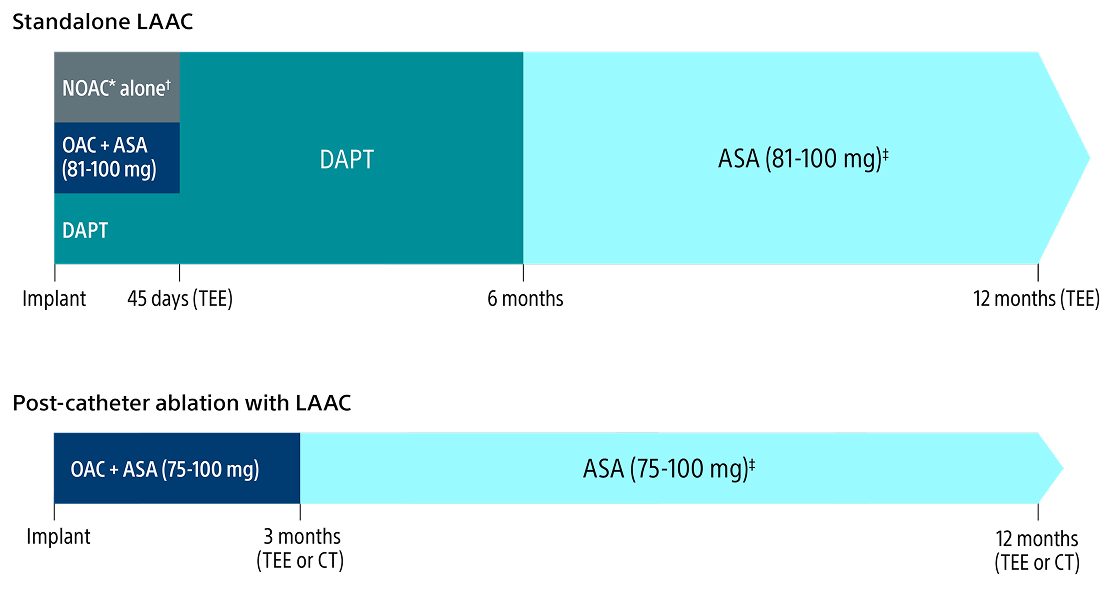
* Excludes Warfarin.
† Pre-procedure ASA is per physician discretion if the physician intends to prescribe NOAC alone for the patient post-procedure.
‡ Continued indefinitely.
Top medical institutions across the country perform the WATCHMAN Implant procedure
- More than 850 major medical centers across the U.S. are certified to implant WATCHMAN
- The WATCHMAN physician training program involves multiple phases including didactic training, imaging training, training in patient selection, device selection, complication management, and optional physician proctoring.
- Individual physicians and/or the collective physician team must be proficient in transseptal skills prior to entering the WATCHMAN training program.
Explore concomitant AFib ablation and WATCHMAN Implant procedures
References:
1. Blackshear JL., Odell JA. Annals of Thoracic Surg. 1996; 61: 755-759.
2. Saliba, W. et al. JACC EP, May 2023. Bench testing or pre-clinical study results may not necessarily be indicative of clinical performance. N=12 in a pre-clinical canine study.
3. Bench study performed under CT by Boston Scientific. Data on file.
