A dual approach to patient care
Performing an AFib ablation and implanting a WATCHMAN Left Atrial Appendage Closure (LAAC) Device in a single procedural event offers a comprehensive solution for addressing atrial fibrillation (AFib) progression, rhythm control, and stroke risk reduction. This approach not only enhances patient satisfaction by alleviating concerns associated with AFib but also allows patients to avoid multiple procedures and potentially discontinue oral anticoagulation therapy earlier.
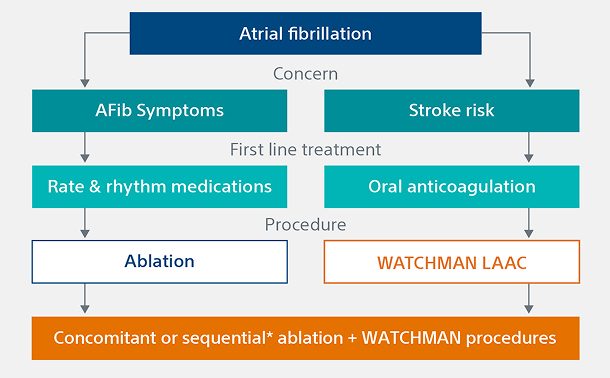
Comparing ablation vs. LAAC treatment options
AFib ablation and LAAC can offer complementary roles in managing AFib. For patients needing rhythm control and/or freedom from oral anticoagulants (OACs), these procedures may provide a comprehensive treatment strategy.
AFib ablation
Address AFib symptoms and achieve rhythm control with multiple ablation modalities, including:

Thermal ablation
Uses extreme heat or cold to create scar tissue and blocks errant electrical signals. This modality may inadvertently affect adjacent tissues.

Pulsed field ablation (PFA)
Utilizes rapid energy pulses to precisely target arrhythmogenic cardiac areas while minimizing collateral tissue damage.
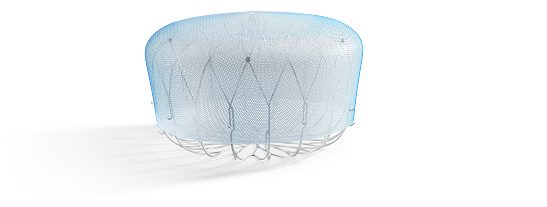
WATCHMAN™ Left Atrial Appendage Closure (LAAC) Implant
The WATCHMAN Implant is a minimally invasive, one-time procedure designed to reduce the risk of strokes that originate in the LAA, offering an alternative to long-term oral anticoagulation therapy and eliminating the risk of long-term OAC-related bleeding complications.
The journey to a combined procedure
As clinical trials broaden AFib patient and treatment indications, clinicians can feel more confident when recommending the WATCHMAN Implant for stroke protection.
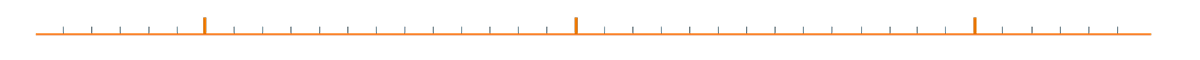
October 2024
CMS expands procedure reimbursement
The Centers for Medicare and Medicaid Services (CMS) introduced a new MS-DRG in October 2024, allowing cost coverage for concomitant AFib ablation and WATCHMAN procedures.1
November 2024
Positive OPTION results announced
Data from the OPTION clinical trial revealed that the WATCHMAN FLX device offers comparable safety and efficacy to OAC in stroke risk reduction for post-ablation patients.2†
April 2025
OPTION sub-analysis published
In a sub-analysis of the OPTION trial, the WATCHMAN FLX device demonstrated similar efficacy to OACs (95% DOACs), while significantly reducing bleeding risks irrespective of concomitant or sequential LAAC following an ablation, providing an option for patients seeking an alternative to long-term anticoagulation regardless of the timing of LAAC relative to the ablation.3
WATCHMAN procedure options for comprehensive AFib management
For AFib patients suitable for the WATCHMAN LAAC Device, implanting physicians may suggest one of three procedure options, depending on their eligibility for an AFib ablation and the physician's discretion. Ensure your patients are informed about potential procedure options before their AFib consultation.
 Permanent implant
Permanent implant
Standalone
A WATCHMAN device is implanted in a single procedure.
 Minimally invasive
Minimally invasive
Concomitant
An AFib ablation is performed, and a WATCHMAN device is implanted in a single procedural setting.
 1 day or less average hospital stay
1 day or less average hospital stay
Sequential
An AFib ablation is performed, and a WATCHMAN device is implanted in a later procedure.*
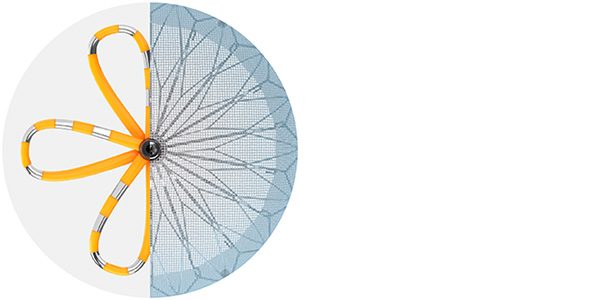
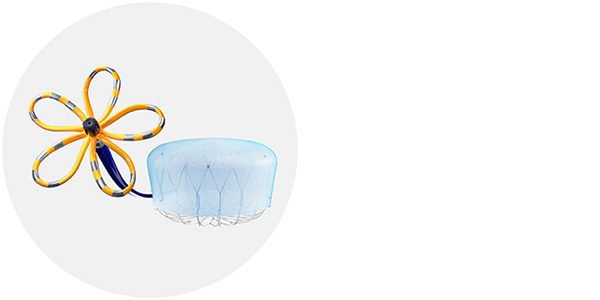
Note: The FARAPULSE PFA Catheter is pictured as representative example for AFib ablation, though any modality may be used.
Boston Scientific provides two industry-leading solutions to help patients manage AFib symptoms and protect against stroke:

FARAPULSE™ Pulsed Field Ablation System
The world's clinical leader in Pulsed Field Ablation (PFA), supported by the world's largest safety registry.4 Treats rate and rhythm symptoms in AFib patients with minimal risk of post-procedural complications.
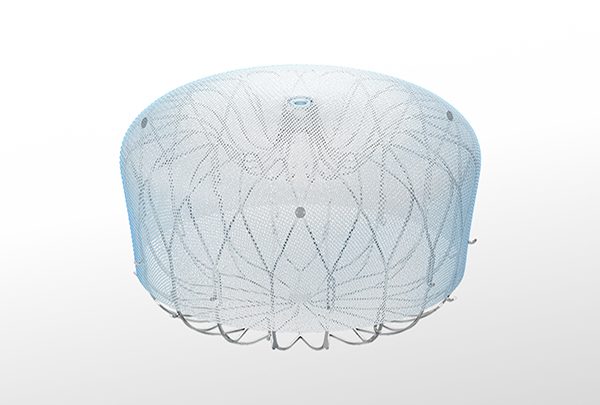
The WATCHMAN Implant
As the most-studied and implanted LAAC device globally, WATCHMAN delivers a lifetime of stroke protection, without the bleeding risks associated with long-term OAC therapy.
See WATCHMAN clinical evidence
WATCHMAN FLX is an FDA approved device being studied for an expanded indication as a first line therapy vs NOAC for NVAF patients. The use of WATCHMAN or WATCHMAN FLX as a first-line therapy for stroke risk reduction in NVAF patients is considered investigational.
*In the OPTION trial, sequential LAAC was a minimum of 90 days (as a protocol-driven blanking period) and less than 6 months post-AF ablation.
†Thermal AFib ablation only.
References
- FY25 IPPS Final Rule
- Wazni O, et al. Randomized Comparison of Left Atrial Appendage Closure with Oral Anticoagulation After Catheter Ablation for Atrial Fibrillation. Late Breaking Clinical Trial, American Heart Association 2024.
- Saliba W, et al. Comparison of Left Atrial Appendage Closure and Oral Anticoagulation after Catheter Ablation for Atrial Fibrillation Concomitant and Sequential Cohorts of the OPTION Randomized Controlled Trial. Late Breaking Clinical Trial, AF Symposium 2025
- Ekanem E, Neuzil P, Reichlin T, et al. Safety of pulsed field ablation in more than 17,000 patients with atrial fibrillation in the MANIFEST-17K study. Nat Med. 2024;30:2020–2029. https://doi.org/10.1038/s41591-024-03114-3
