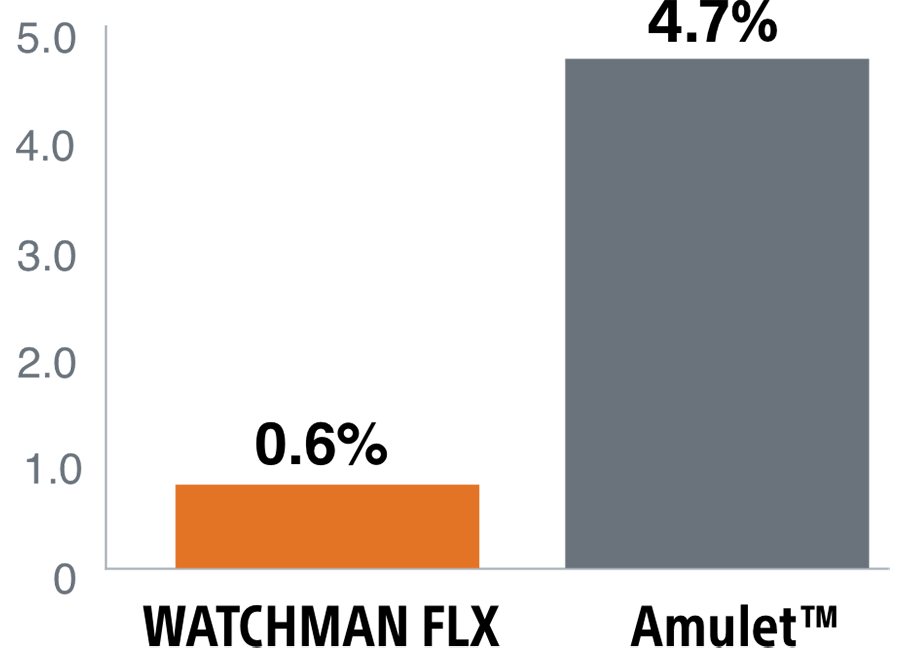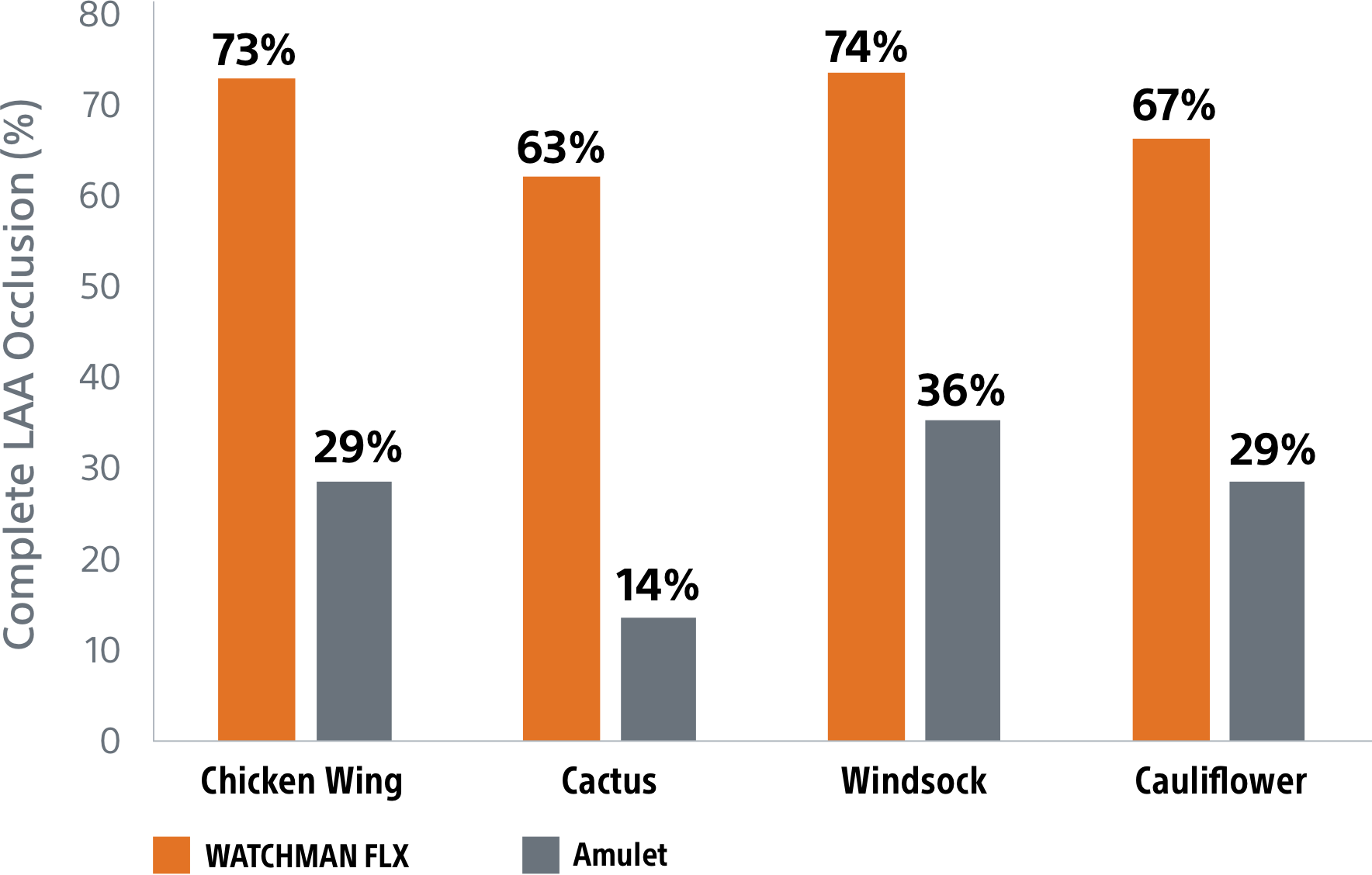WATCHMAN FLXTM
Left Atrial Appendage Closure Device
Setting the standard
Built on the most studied LAAC device in the world – WATCHMAN FLX delivers market-leading safety, simplicity and seal.
Safety above all
Proven to deliver safety like no other, the WATCHMAN FLX device leads the industry with a low major adverse event rate across multiple studies.
Simplicity without compromise
To reduce procedure time and treat the widest range of LAA anatomies, trust the device that delivers an unmatched implant experience. WATCHMAN FLX is simply the easiest and most intuitive LAAC device to use with near universal procedural success across multiple studies and large patient populations.
Unmatched procedural success rates
| Clinical Trial | Real-world Study |
| 100% | Alster Registry4 n = 164 |
| 100% | SWISS-APERO5 n = 110 |
| 99% | PINNACLE FLX1 n = 400 |
| 99% | Danish Study6 n = 91 |
| 99% | FLXibility Registry7 n = 300 |
| 98% | SURPASS2 n = 66,894 |
| 98% | NCDR WATCMAN FLX vs. Legacy WATCHMAN8 n = 27,103 |
| 97% | PINNACLE FLX Failed Legacy WATCHMAN9 n = 88 |
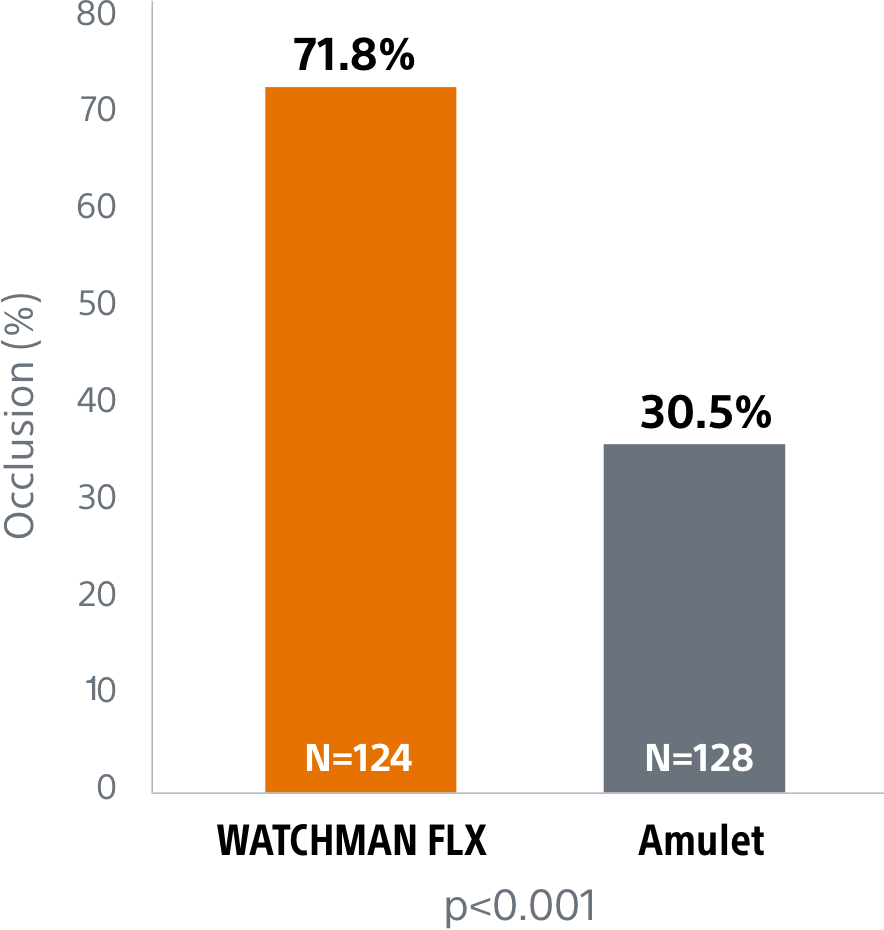
Superior complete occlusion** rates by LAA anatomy10
Seal with confidence
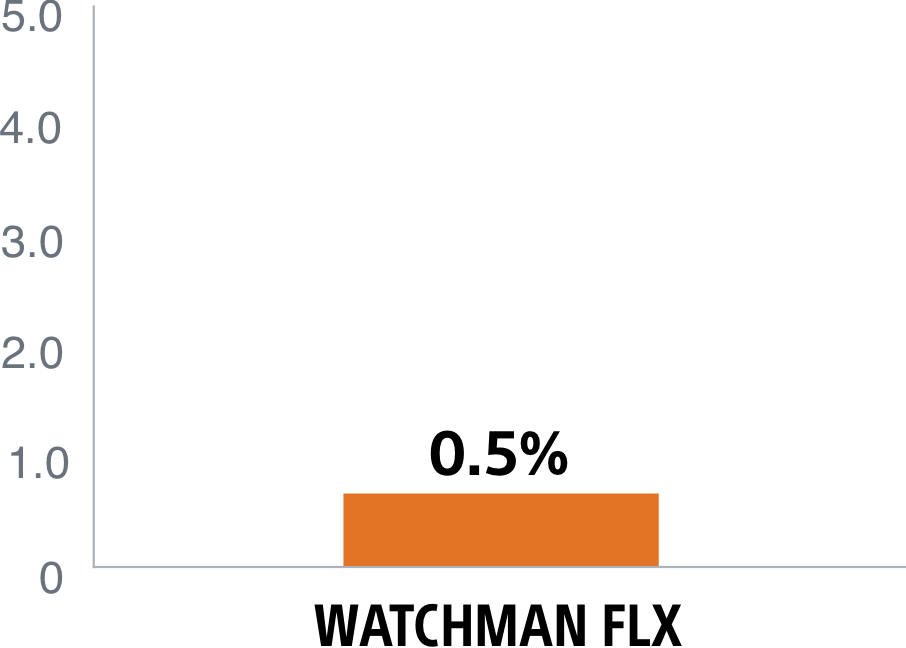
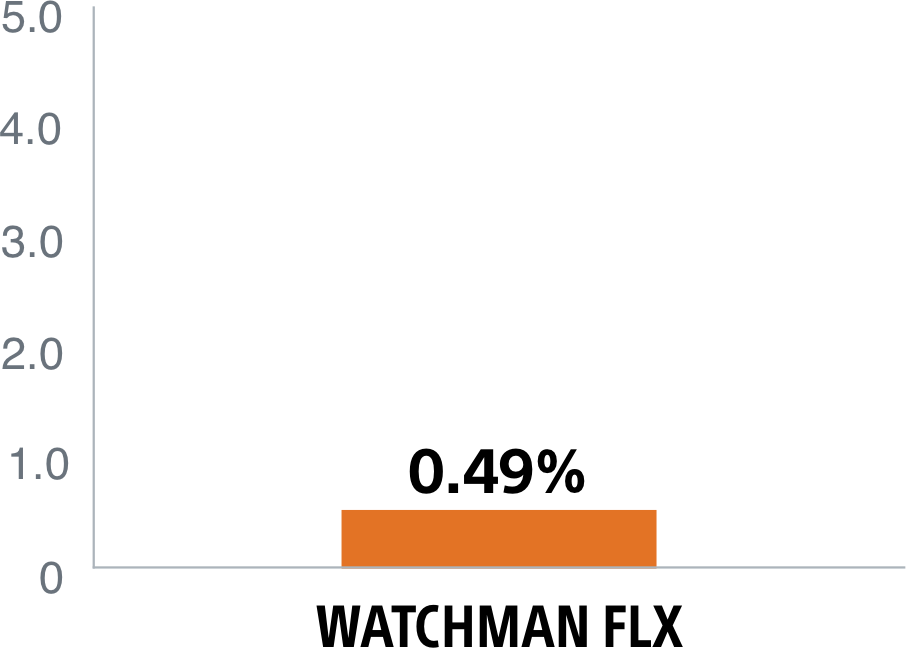
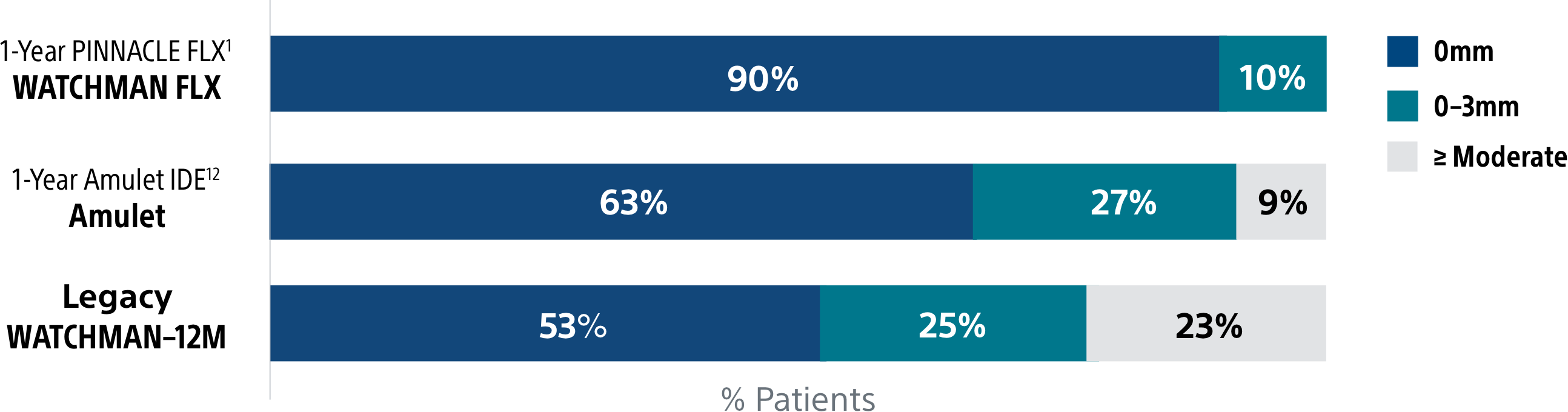
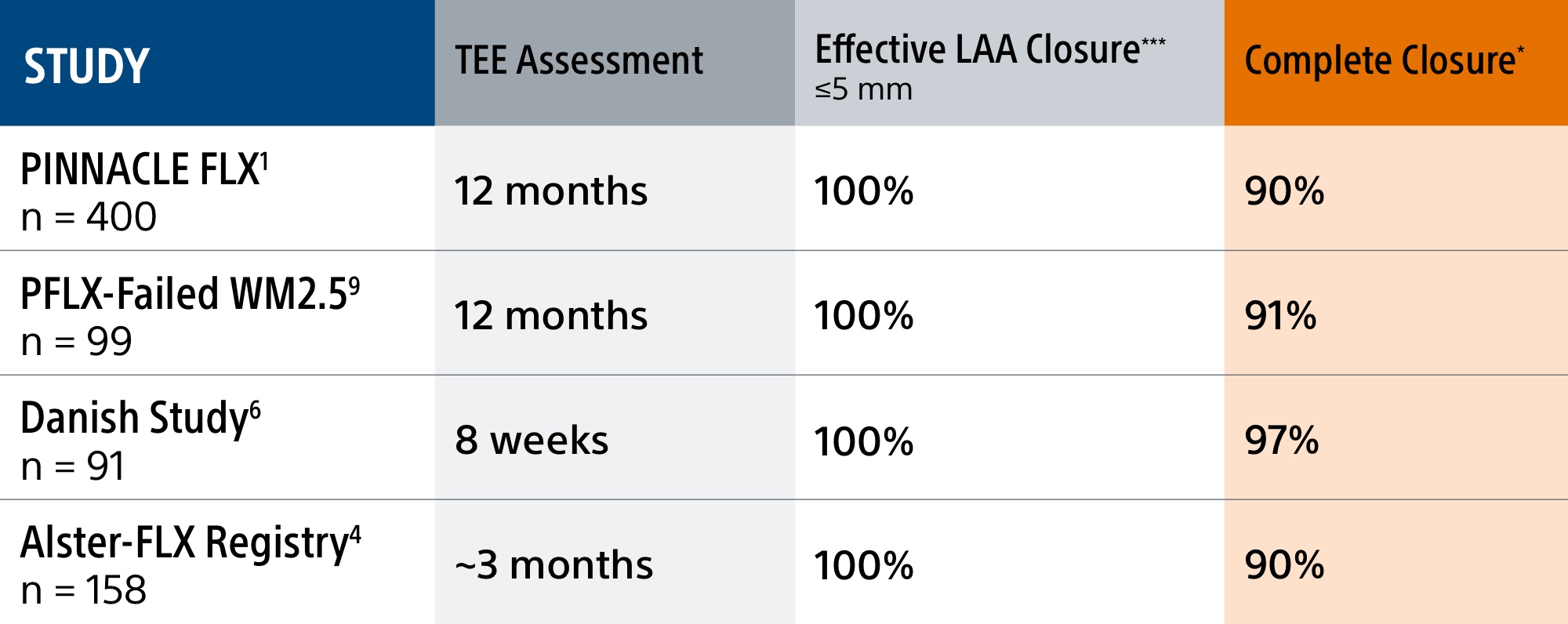
The WATCHMAN FLX device's enhanced, single component design helps simplify placement for a quicker, more complete seal. It's the only device with a demonstrated complete closure* rate of 90%1, setting the LAAC therapy standard.
Complete closure* rate of WATCHMAN FLX compared to Amulet and Legacy WATCHMAN
*Complete closure is defined as no visible peri-device leak (PDL) via TEE
**Complete LAA occlusion defined as no visible peri-device leak (PDL) and absence of contrast patency in the distal LAA (LAA/left atrium Hounsfield ratio <0.25)
***Effective closure is defined as less than or equal to 5mm residual leak
WATCHMAN FLX DAPT approval
FDA approves use of DAPT or OAC immediately following implant with WATCHMAN FLX device.
Learn more about WATCHMAN FLX
Access resources
Access WATCHMAN FLX device resources by visiting our WATCHMAN Download Center
Rep visit
Connect with a local representative to learn more about the WATCHMAN FLX device
Sources:
- Kar, S et al. Circulation. 2021;143:1754–1762.
- Late Breaking Clinical Trial at CRT 2023, Presented by Samir Kapdia.
- Della Rocca et al, Procedural and Short-Term Follow-Up Outcomes of Amplatzer Amulet Occluder versus WATCHMAN FLX Device: A Meta-Analysis, Heart Rhythm 2022.
- Bergmann et al. Alster-Registry, Presented ePCR 2021.
- Galea, SWISS-APERO Trial, CIRCULATION, 2021.
- Korsholm, et al. WATCHMAN FLX First Experience, JACC, 2020.
- Betts et al. Poster Presentation HRS, 2021.
- Freeman et al. LBCT Presented at HRS 2022.
- Ellis, Heart Rhythm, 2021.
- Korsholm et al. Journal of Interventional Cardiac Electrophysiology, 2022.
- Reddy VY, et al. JACC 2017; 70(24): 2964-2975.
- Lakkireddy, D et al. Circulation. 2021;144:1543–1552.