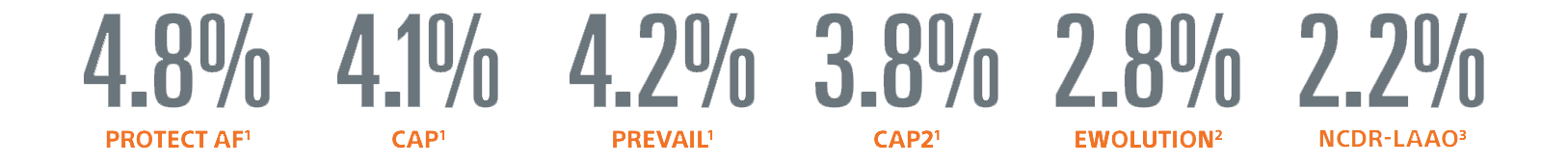Procedural safety
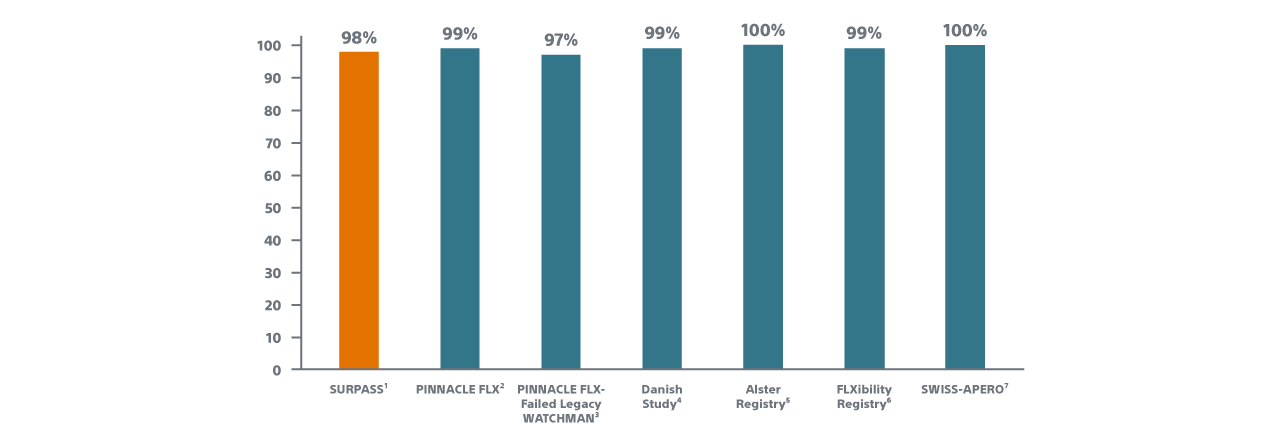
PROCEDURAL SUCCESS
*Procedure success defined as successful delivery and release of a WATCHMAN FLX device into the LAA.
Reported N values on this slide are those of attempted implants. All cancelled procedures are excluded from this analysis.
1. Late Breaking Clinical Trial at CRT 2022, Presented by Dr. Samir Kapadia.
2. Kar, PINNACLE FLX; 12 Month Outcomes, CIRCULATION, 2021.
3. Ellis, Structural Heart, 2021.
4. Korsholm, WM FLX First Experience, JACC, 2020.
5. Bergmann, Alster-Registry, Presented ePCR 2021.
6. Betts, Poster Presentation HRS, 2021.
7. Galea, SWISS-APERO Trial, CIRCULATION, 2021.
REFERENCES
* Implant success defined as deployment and release of the device into the LAA; no leak ≥ 5 mm
1. WATCHMAN FDA Panel Sponsor Presentation. Oct 2014.
2. Boersma, et al, Heart Rhythm, Vol 14, No 9. September 2017.
3. Freeman, JACC, March 2020, Vol 75, No. 13, 2020.
4. Doshi SK, et al. PINNACLE FLX Results Presented at HRS 2020.
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.