WATCHMAN LAA closure devices
Clinically proven outcomes
SURPASS Data Update
The SURPASS Analysis of the NCDR LAAO Registry includes the largest number of commercial Watchman FLX patients to date.This data reinforces the outstanding safety of WATCHMAN FLX with a 0.37% major procedural adverse event rate at 7 days or hospital discharge, and a 0.28% ischaemic stroke rate through 45 days in >16,000 real world NVAF patients.
Key Safety Endpoint within 7 days or Hospital discharge:
- The very low o.37% procedural adverse event rate in 16, 048 commercial patients confirms the trusted safety profile of Watchman FLX in a broader clinical practice setting.
- 0.32% of patients had a pericardial effusion requiring intervention at discharge.
- SURPASS data demonstrates low adverse events and a 0.28% ischeamic stroke rate at 45 days.
- Excellent procedural success with 98% of patients implanted (N= 16048/16446)
These data reinforce the high procedural success and low major procedure-related complication rate of Watchman FLX in this large real-world experience.
82% complete seal at 45 days in SURPASS, comparable to PINNACLE FLX complete seal at 45 days.
Long-term results demonstrate WATCHMAN reduces stroke, bleeding and mortality vs warfarin
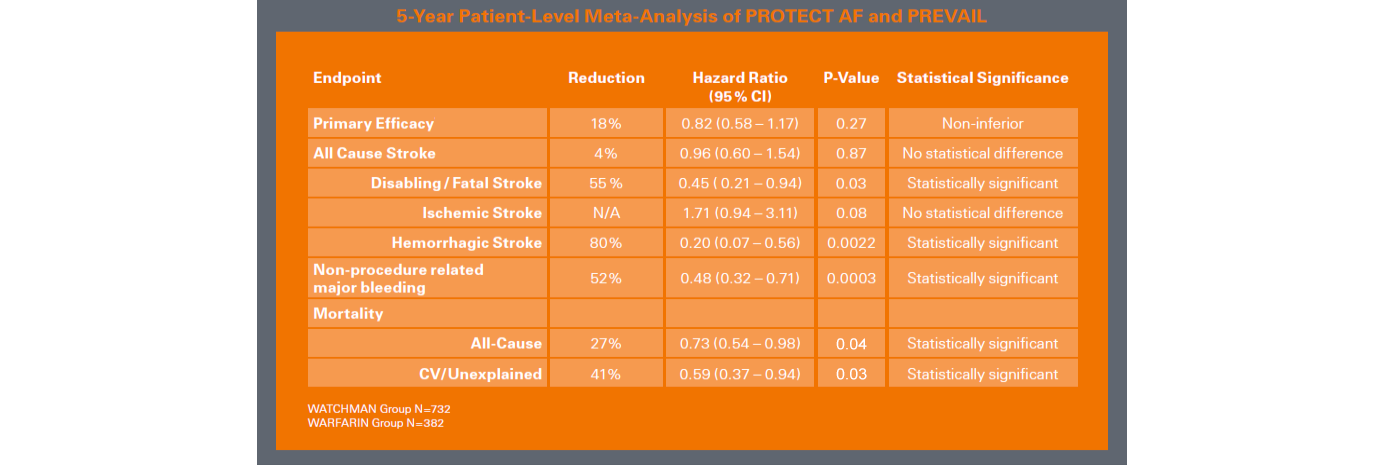
Long-term results from a patient-level meta-analysis of the PROTECT AF (2,717 pt yrs) and PREVAIL (1,626 pt yrs) trials demonstrated that WATCHMAN offered, after 5 years of follow up1:
- Comparable stroke risk prevention vs. warfarin.
- Statistically significant reductions in disabling and fatal stroke (55%) and significant mortality benefit (41% risk reduction for CV death and 27% reduction in all cause mortality) vs warfarin.
- At 6 months post procedure, the patient group with LAA closure showed a reduction of major bleeding events vs warfarin of 72% (1.0 vs 3.5, P<0.001)2.
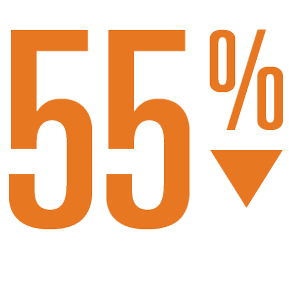
Relative Risk Reduction
In Disabling and Fatal Strokes1
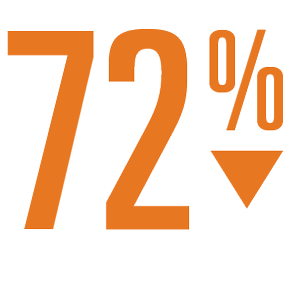
Relative Risk Reduction
In Major Bleeding 6-months Post Implant2
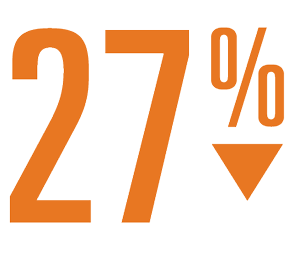
Relative Risk Reduction
In All-Cause Mortality1
SURPASS SAME DAY DISCHARGE (SDD) Data
- SDD patients were significantly younger and healthier than LDD patients. They also had lower CHA2DS2-VASc and HAS-BLED scores.
- SDD patients had a 2.78% rate of major adverse events through 45 days. Later Day Discharge (LDD) patients had a 5.22% rate.
- Both SDD and LDD patients demonstrated >80% complete closure at 45 days (SDD = 83.3%, LDD = 81.7%)
EWOLUTION two-year results confirm that
WATCHMAN is effective in a real world setting
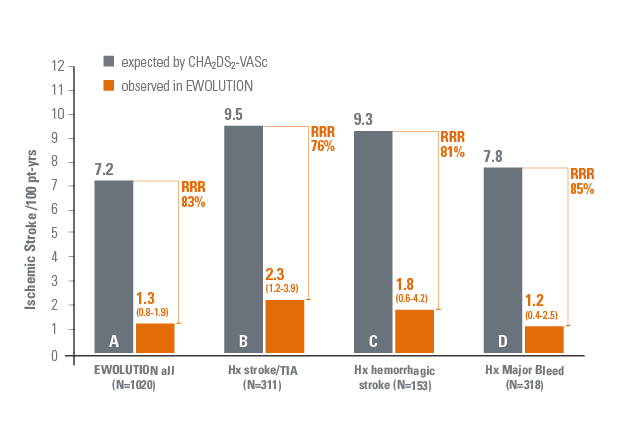
- EWOLUTION, the largest prospective real-life study on WATCHMAN, showed a high success of implant and sealing, with low procedural adverse event rates3 in 1020 patients studied.
- > 70% of the patients in the study were deemed unsuitable for any oral anti-coagulation.3
- Two-year results confirm that WATCHMAN is safe and effective showing:
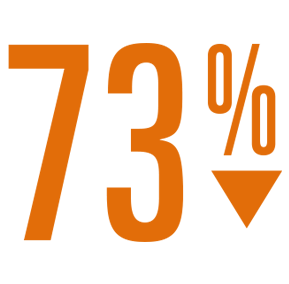
- 83% reduction in ischemic stroke rate (1.3 per 100 pt-yrs)4 as compared to the expected rate with no therapy.5
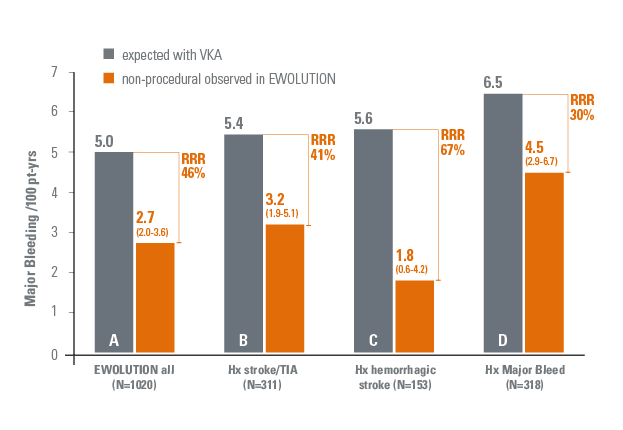
- 46% reduction in non-procedural major bleeding rate (2.7 per 100 pt-yrs)4 compared to the expected rate with warfarin.6
- 83% reduction in ischemic stroke rate (1.3 per 100 pt-yrs)4 as compared to the expected rate with no therapy.5
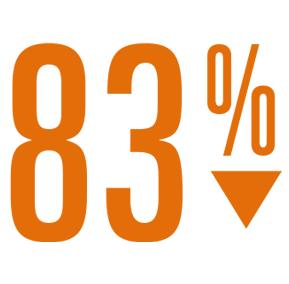
Relative Risk Reduction
IIschemic Stroke Rate
vs expected with no therapy4
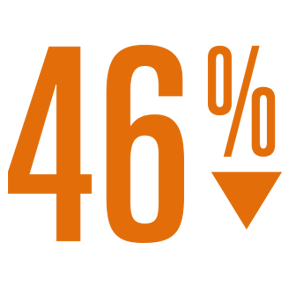
Relative Risk Reduction
Non-procedural, major bleeding
vs expected with warfarin4
of 1020 patient studied
unsuitable for OAC therapy3
LAA closure with WATCHMAN proves to be an effective solution for stroke risk reduction in both high and low risk patients
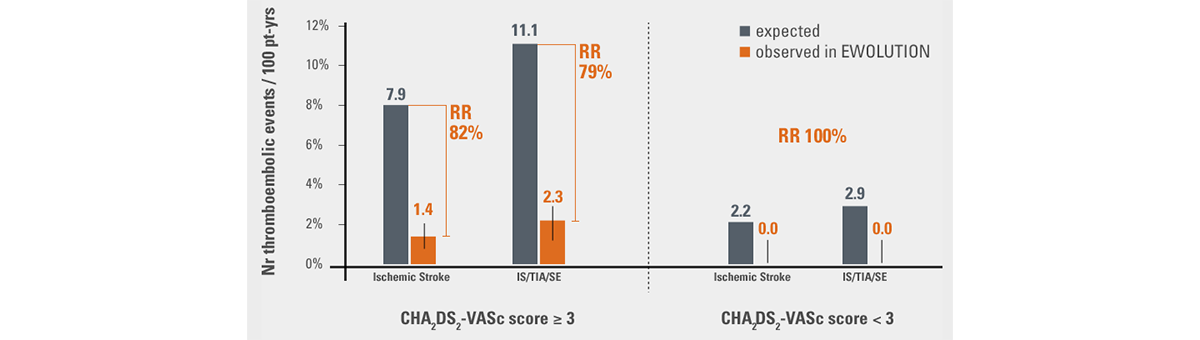
- In patients with CHA2DS2-VASc ≥ 3 an 82% reduction in ischemic stroke rate4 was observed vs. expected
- No stroke occurred in patients with CHA2DS2-VASc < 3, resulting in 100% relative risk reduction4
REFERENCES
* Effectiveness in stroke reduction vs. estimated in the absence of therapy for comparable CHA2DS2-VASc scores based on Friberg et al. EHJ 2012
# Two strokes in PREVAIL are excluded because the baseline MRS score was unavailable 1 Reddy VY et al., 5-Year Outcomes After Left Atrial Appendage Closure From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017 Dec 19;70(24):2964-2975.
1. Reddy VY, Doshi SK, Kar S, et al., 5-Year Outcomes After Left Atrial Appendage Closure From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017 Dec 19;70(24):2964-2975.
2. Price MJ, Reddy VY, Valderrábano M, et al. Bleeding outcomes after left atrial appendage closure compared with long-term warfarin: a pooled, patient-level analysis of the WATCHMAN randomized trial experience. JACC Cardiovasc Interv. 2015;8(15):1925-1932.
3. Boersma LV et al., Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017 Sep;14(9):1302-1308.
4. Boersma LV et al., Stroke, bleeding and mortality of WATCHMAN Left Atrial Appendage Closure in Patients with or without Contraindication to Oral Anticoagulation: 2-year final outcome data of the EWOLUTION Study. Presented at EHRA 2018.
5. Friberg L, et al., Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. European Heart Journal (2012) 33, 1500 -1510.
6. Lip GYK, et al., Comparative Validation of a Novel Risk Score for Predicting Bleeding Risk in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol. 2011 Jan 11;57(2):173-80.
7. Price MJ, et al., Bleeding outcomes after left atrial appendage closure compared with long-term warfarin. JACC Cardiovasc Interv. 2015;8(15):1925-1932.
8. Reddy VY, et al., Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). JACC.2013 Jun 25;61(25):2551-6
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.