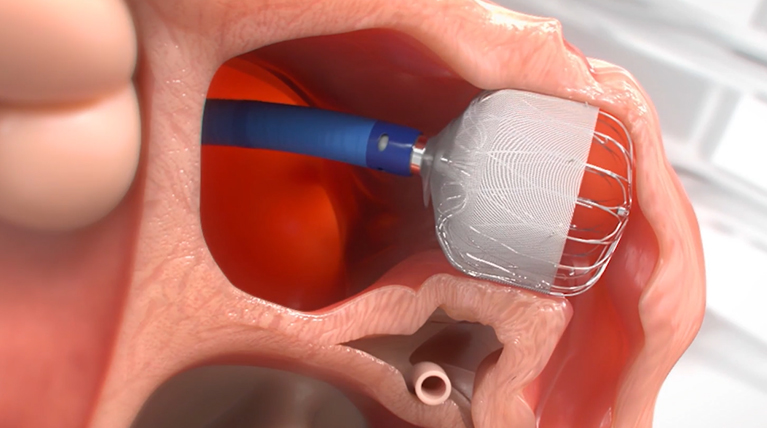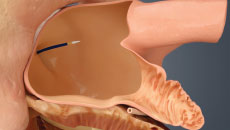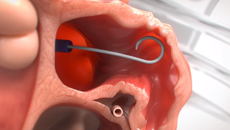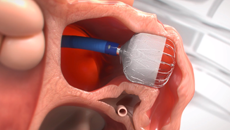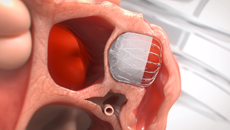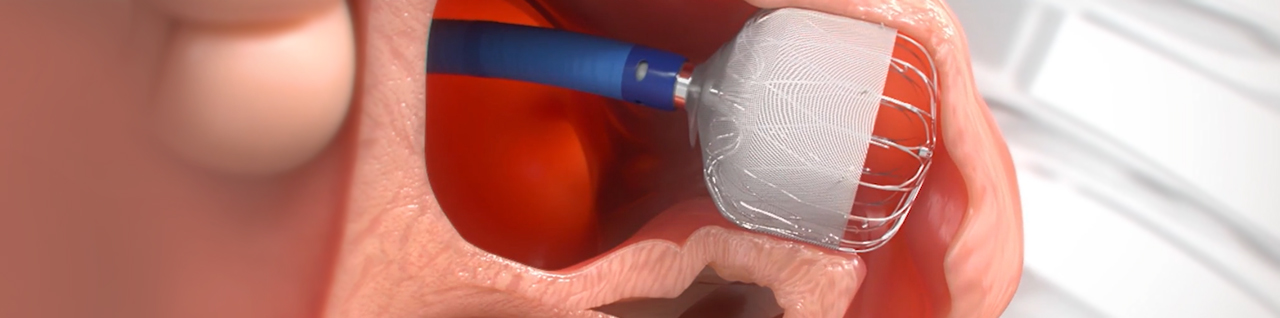
Get Informed About the WATCHMAN FLXTM Implant Procedure

MINIMALLY INVASIVE

PERMANENT IMPLANT

TYPICAL PROCEDURE IS LESS THAN 1 HOUR

24 HOUR AVERAGE HOSPITAL STAY
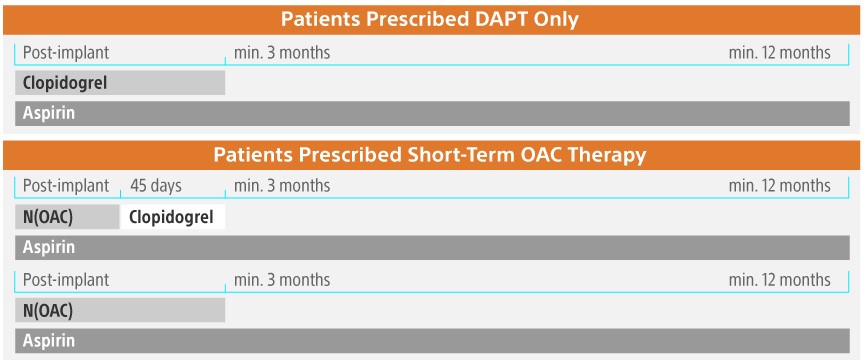
Post-Implant Drug Regimen
Post-implant medication is at the the discretion of each physician and is subject to the patient's specific condition and medical history. Boston Scientific does provide general guidelines in the instructions for use (IFU) of the device, based on the robust clinical results observed in the RCTs and the EWOLUTION registry.
REFERENCES
* Implant success defined as deployment and release of the device into the LAA; no leak ≥ 5 mm
1. Boersma LVA et al. Implant Success and Safety of Left device: periprocedural outcomes from the EWOLUTION Registry. Eur Heart J 2016;37(31):2465-74.
2. INTERIM POST-MARKET SURVEILLANCE STUDY REPORT on EWOLUTION May 5, 2017
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.