LAA Closure
A one-time Solution for Reducing the Risk of Stroke
Most studied LAAC device in the world
With over 20 years of LAAC innovation and experience, WATCHMAN has changed the lives of over 300,000 patients and has advanced the standard of care with proven, safe, and trusted patient outcomes. WATCHMAN FLX, the only device custom-engineered for the LAA, is a generation ahead and in a class all on its own.
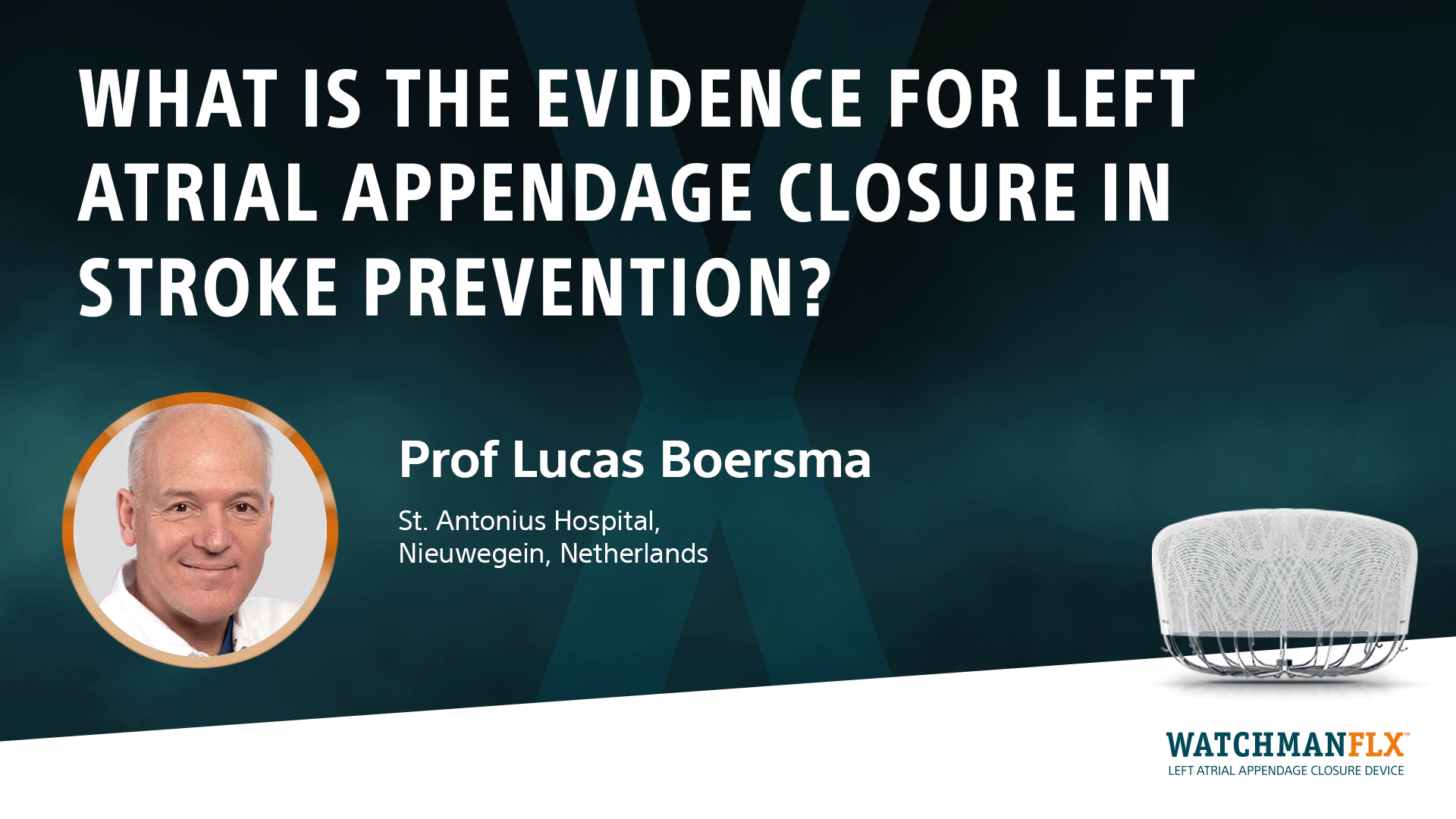
Proven Procedure
Learn about the efficacy results of WATCHMAN in clinical trials of patients with non-valvular AF, eligible or with contraindication to OAC therapy
REFERENCES
* In non-valvular AF Patients
1. Reddy VY, 5-Year Outcomes After Left Atrial Appendage Closure From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol. 2017 Dec 19;70(24):2964-2975.
2. Doshi SK, et al. PINNACLE FLX Results Presented at HRS 2020.
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.