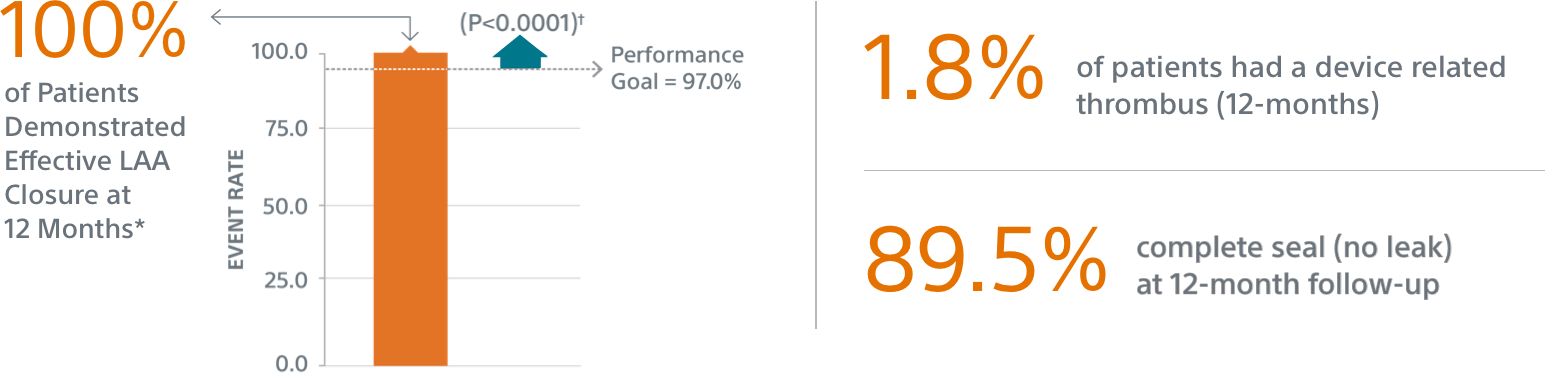PINNACLE FLX
PINNACLE FLX full study design
The U.S. IDE Trial was designed to establish the procedural safety and left atrial appendage closure efficacy of WATCHMAN FLX™ LAAC DEVICE
- 400 patient, 29 US site, single arm, non-randomized trial evaluating WATCHMAN FLX for non-inferiority to safety and efficacy performance goals based on the WATCHMAN™ device.
- Follow-up: 45 days (+TEE), 6 months, 12 months (+TEE), 18 months, and 24 months
- Patient Characteristics: Average CHA2DS2-VASc of 4.2 ± 1.5, Average HAS-BLED of 2.0 ± 1.0
- Post Implant Drug Regimen: NOAC/ASA for 45 days, Clopidogrel/ASA to 6 months, ASA post 6 months
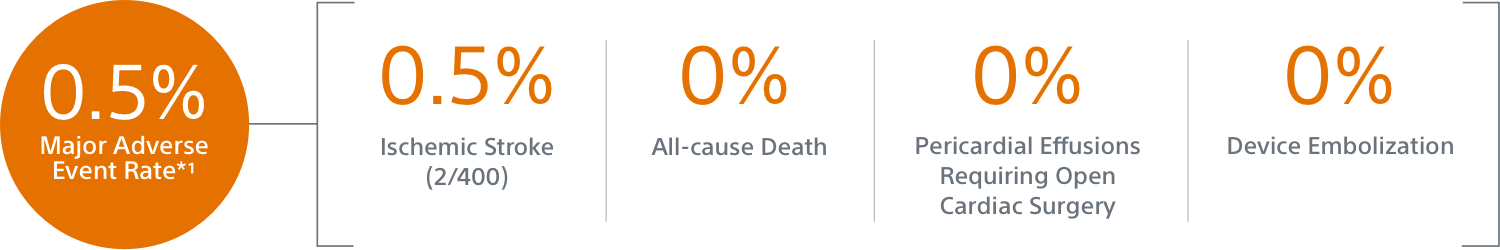
- Primary Safety Endpoint: All-cause death, ischemic stroke, systemic embolism, or device- or procedure-related adverse events requiring surgery or major endovascular intervention within 7 days following the procedure or by hospital discharge, whichever is later.
- Primary Efficacy Endpoint: The rate of effective LAA closure defined as any peri-device flow ≤5mm demonstrated by TEE at 12 months
- Secondary Efficacy Endpoint: The occurrence of ischemic stroke or systemic embolism at 24 months from the time of enrollment
- Inclusion/exclusion criteria is consistent with WATCHMAN clinical study inclusion/exclusion criteria. Patients must be eligible for short-term NOAC vs warfarin in previous clinical studies.
Advance safety1
Proven safety outcomes
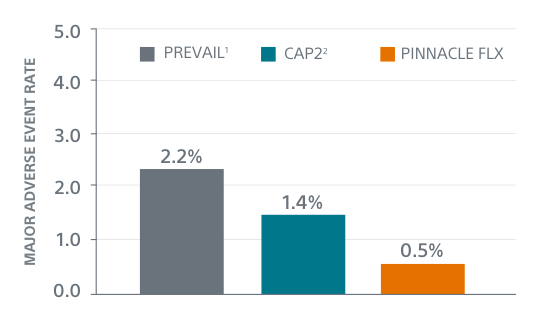
The low 0.5% event rate demonstrates the enhanced safety profile of the WATCHMAN FLX LAAC device, showing a statistically significant difference to the performance goal set for similar safety endpoints in the PREVAIL Trial and CAP2 Registry.*
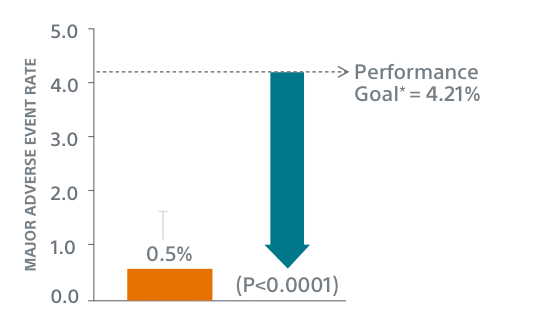
* Based on the combined rate observed in PREVAIL(3) and CAP2(4), plus a clinically acceptable delta.
1. Holmes, DR. et al. (2014). J AM Coll Cardiol 64(1): 1-12
2. Holmes, DR et al. JACC 2019
* PREVAIL(3) & CAP2(4) studied the WATCHMAN LAAC Device
** Occurrence of one of the following events between the time of implant and within 7 days following the procedure or by hospital discharge, whichever is later: all-cause death, ischemic stroke, systemic embolism, or device or procedure related events requiring open cardiac surgery or major endovascular intervention
Advance procedural performance1
Proven efficacy outcomes
The WATCHMAN FLX LAAC device is designed for enhanced LAA closure which was demonstrated with 100% rate of effective LAA closure at 12 months.*
* LAA closure at 12 months is defined as any peri-device flow with jet size ≤5mm per core laboratory-assessed TEE
† Performance goal based on the rates observed in PREVAIL(3) and CAP2(4), minus a clinically relevant delta
Expand the treatable patient population1
Designed to treat the widest range of patient anatomies
Procedure performance

Broader range of anatomies†

6.6% of enrolled patients in PINNACLE FLX were previous WATCHMAN screen fails or case aborts, and 97% of those patients were successfully implanted with WATCHMAN FLX5
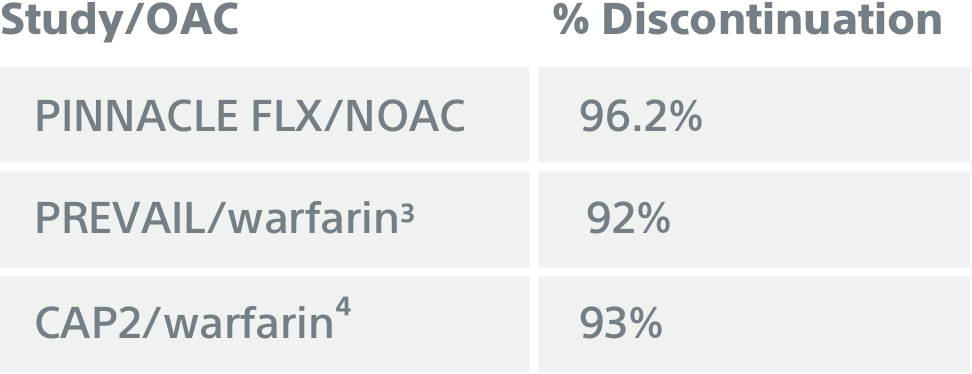
NOAC discontinuation

* Procedure success defined as successful delivery and release of a WATCHMAN FLX device into the LAA
† Measured maximum LAA ostium width and/or deployed closure device diameter must be ≥ 14.0 mm and ≤ 31.5 mm to accommodate available Closure Device sizes. LAA depth should be approximately half the labeled implant diameter or longer
‡ This figure includes both the main cohort, and roll-in patients (totaling 452 implants)
1. Kar S, et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation. 2021;143:1754-1762.
2. Holmes, DR., et al, (2014). J AM Coll Cardiol 64(1): 1-12.
3. Holmes, DR., et al, JACC 2019.4. Ellis et al. Presented at AF Symposium 2020.
4. Ellis et al. Presented at AF Symposium 2020.