Shared decision‑making
An evidence-based cornerstone of WATCHMAN
What is shared decision-making?
- Shared decision-making is a collaborative process that allows patients and their physicians to make treatment decisions together
- The process considers the best scientific evidence available, as well as the patients' values and preferences1
The role of shared decision-making in LAAC procedures2
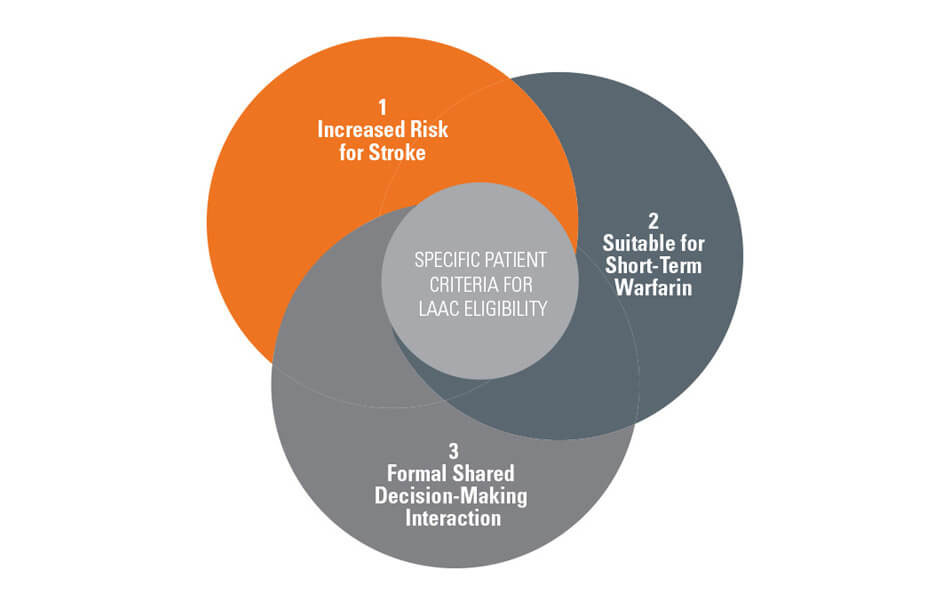
- A formal shared decision-making interaction with an independent non-interventional physician using an evidence-based decision tool is recommended for patients with non-valvular atrial fibrillation (NVAF) prior to left atrial appendage closure (LAAC)
- The shared decision-making interaction must also be documented in the medical record
- The patient must be suitable for short-term warfarin but deemed unable to take long-term oral anticoagulation (OAC) following the conclusion of shared decision-making, as LAAC is only covered as a second-line therapy to oral anticoagulants
Why is shared decision-making important?
The Centers for Medicare and Medicaid Services (CMS) encourages the use of an evidence-based tool in discussions about LAAC therapy because it helps:
- Compare the risks and benefits to oral anticoagulants
- Determine and document the appropriateness of LAAC as a non-pharmacological treatment option
- Uncover barriers to change that include physical pain, emotional difficulties, financial concerns and lack of confidence in one's ability to change
- Address any barriers to develop a realistic personal prevention plan with specific and achievable outcomes
How shared decision-making works
The Agency for Healthcare Research and Quality's SHARE approach is a five-step shared decision-making process that compares the benefits and risks of each treatment option through meaningful dialogue about what matters most to the patient.
Using the SHARE approach in your practice3
LAAC patient eligibility
The shared decision-making interaction must be documented in your patient's medical record.
Shared decision-making resources
The following resources will help you have more productive conversations with your patients about the risks and benefits to anticoagulation treatment for non-valvular atrial fibrillation.
National Institute for Health and Care Excellence Patient Decision Aid
References:
- Centers for Medicare & Medicaid Services. Decision memo for percutaneous left atrial appendage (LAA) closure therapy (CAG-00445N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=281&ExpandComments=n&DocID=CAG-00445N&bc=gAAAAAgAAgAAAA%3d%3d&. Published February 8, 2016. Accessed May 20, 2016.
- Barry MJ, Edgman-Levitan S. Shared decision making—the pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781.
- Agency for Healthcare Research and Quality. U.S. Department of Health & Human Services. http://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Accessed May 20, 2016.